Hydrogen Bonding in Water
The article discusses the variations in hydrogen bond length in water depending on temperature and pressure. Hydrogen bonds in D2O are found to be more asymmetric and stronger than in H2O, but recent studies have shown that the hydrogen bonds in both substances are approximately the same length due to compensatory quantum effects. Increasing pressure can force shorter hydrogen bonds, causing the O-H covalent bonds to be elongated. The strength of hydrogen bonds can also be affected by electromagnetic and magnetic effects. While dissociation is a rare event, it may occur during the breakage or formation of a hydrogen bond, or arise transiently due to thermal effects or other molecular interactions.
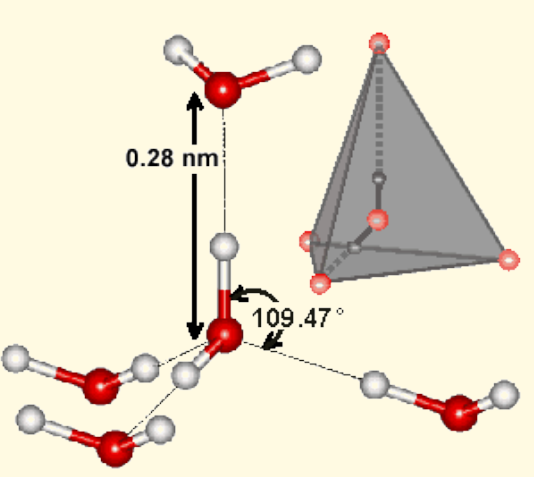
This is relevant as the tetrahedral water molecule is a excellent instance of the tetrahedral tholonic model. Also, it may shed some light on the effects of kinetic energy on water, i.e., ‘structured water’.